Laminin-211 (Lm-211), a heterotrimeric glycoprotein containing the LAMA2 gene product, is a key component of the muscle BL. This laminin links the BL to the cytoskeleton through plasma membrane receptors and forms an extracellular scaffold by polymerization and bonding to other BL proteins. In MDC1A, Lm-411 expression increases in a compensatory fashion but is ineffective, likely because it lacks the Lm-211 receptor-binding and polymerization activities. This opens the door to correcting the BL structural defect by altering the properties of Lm-411 to resemble that of the missing Lm-211.
Restoration of laminin assembly with gain-of-function linker proteins (figures).

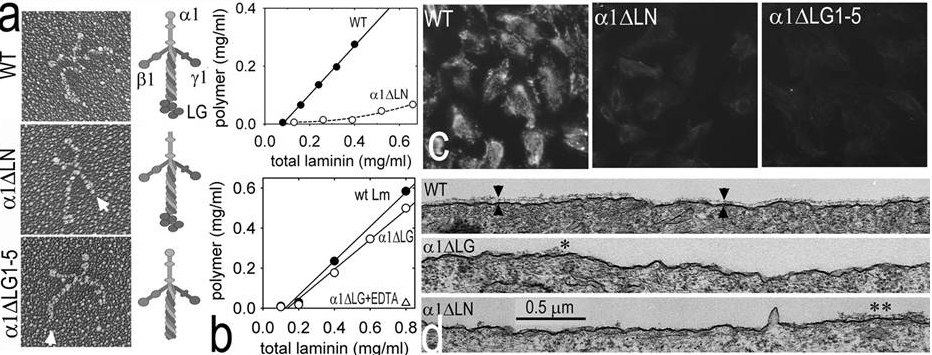
Fig. above: Rotary shadowed electron micrographs of wild-type laminin, laminin without a polymerization LN domain, and without adhesion LG domains are shown. Laminins that cannot polymerize or that cannot adhere to cells are unable to assemble on cell surfaces.
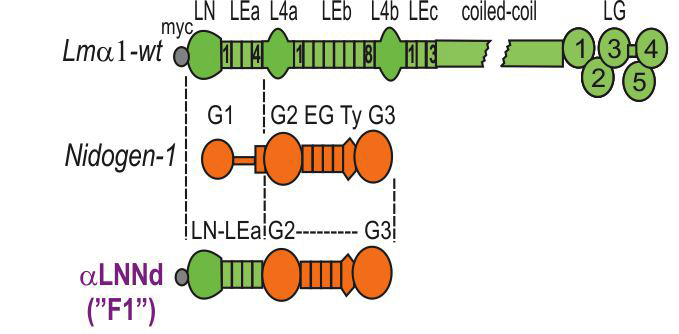
Fig. above: AlphaLNNd was made by fusing the laminin a-subunit LN and adjacent LEa domains (for polymerization) to the G2 (collagen-IV and perlecan-binding), EG, Ty and G3 domain (binds laminin) of nidogen-1. Shown below is miniagrin (mAgrin, mA), a shortened version of agrin that binds to laminins and to cell surface dystroglycan, increasing the affinity of laminin binding to the cell surface. Miniagrin permits laminin-111 with deleted LG domains to bind cells and assemble a BL. These linker proteins hold potential to enable assembly and adhesion and polymerization-deficient laminins. A3 Char-acterization of aLNNd for polymerization.
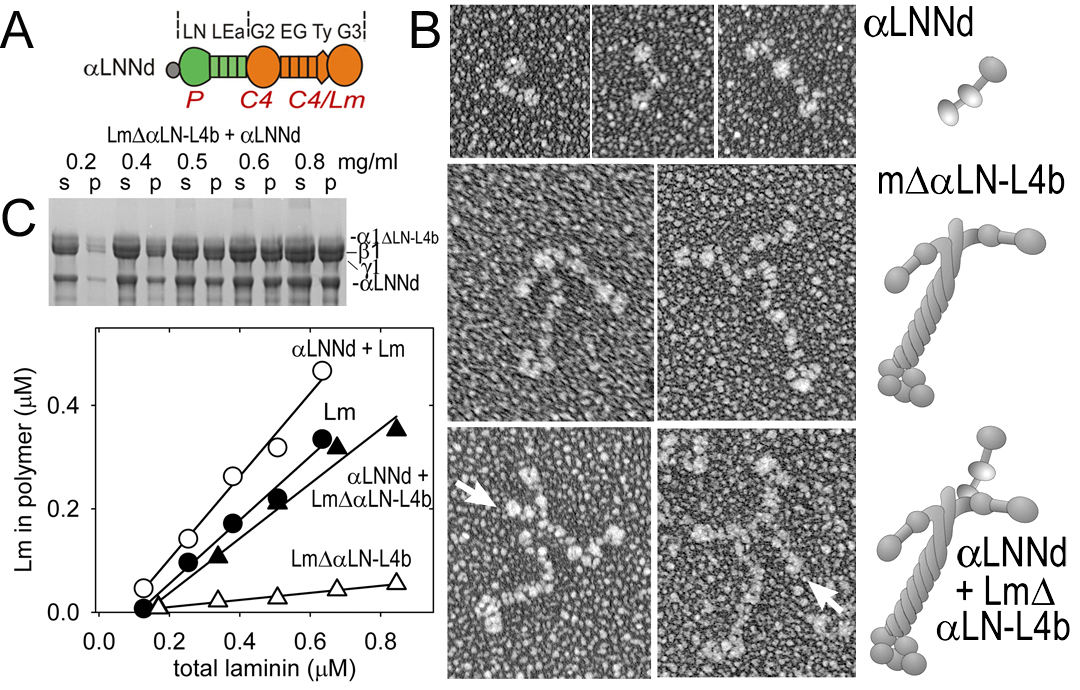
Fig. above: Molecular morphology (EM, rotary shadows) of aLNNd alone, bound to a non-polymerizing laminin, and compared to miniagrin bound to laminin. C. aLNNd restores polymerization activity to a laminin that lacks the critical aLN domain. Below: aLNNd enables assembly of a non-polymerizing laminin on Schwann cells. In contrast, miniagrin enables assembly of a laminin without LG domains. aLNNd + miniagrin enables assembly of a laminin lacking both LN and LG domains.
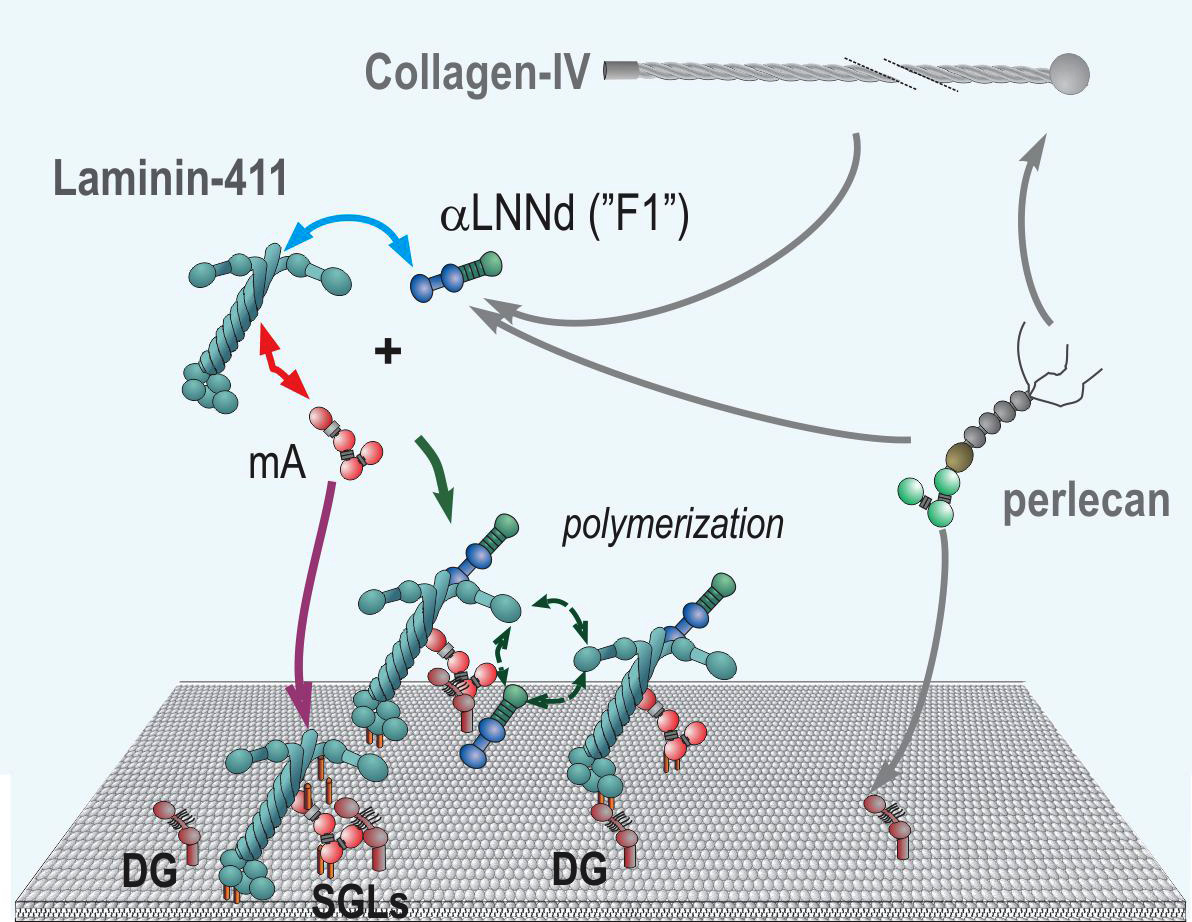
Fig. above: Model of combined alphaLNNd and miniagrin repair of laminin-deficiency through modification of the compensatory laminin-411 that is unable to polymerize and that has weak affinity for the cell surface. AlphaLNNd enables polymerization while miniagrin increases cell surface affinity (note: miniagrin and its effects on mice was described by our collaboration Markus Ruegg (Univ. Basel) - see Moll et al., 1999.)
We are now evaluating the effect of expression of aLNNd in two mouse models of laminin-deficient congenital muscular dystrophy.
MDC1A congenital muscular dystrophy is accompanied by demyelination of peripheral nerves. The BL of the peripheral nerve Schwann cell (endoneurium), like that of muscle, is normally enriched in the polymerizing laminin-211, but also contain the non-polymerizing laminin-411. There are several mouse models that include dy2J (a laminin polymerization defect), dy3K (laminin alpha2 subunit knockout) and the laminin gamma1 hypomorph. The last, which is a new project in our lab, results in mild muscle but severe nerve pathology. This nerve pathology can be recapitulated in cultured dorsal root ganglia that are laminin-deficient.
Mammalian peripheral nerves have axons that are myelinated. A myelinated axon a little like an insulated wire. Myelination prevents low conduction velocities through saltatory conduction between the spinal cord and nerve targets. During late development, Schwann cells (SCs; glial cells derived from the neural crest) undergo "radial axonal sorting" in which they extend cell processes that separate and envelop individual axons. Radial sorting requires laminins-211 and -411 (and can be substituted by laminin-111) following the formation of the endoneurial (Schwann cell) basal lamina.
Loss of myelination but not Schwann cell proliferation in a laminin hypomorphic state: LamC1 floxed conditional knockout mice were bred with Sox2cre mice to generate LamC1 fl/- mice. These mice are hypomorphic with respect to expression of the near-universal Lmg1 subunit. The mice developed a gait abnormality characteristic of mice with a deficiency of the nerve/muscle-specific Lma2 subunit. Sections of sciatic nerve showed the radial sorting defect with amyelination. Unique among laminin mutations, there was no reduction of Schwann cell proliferation.
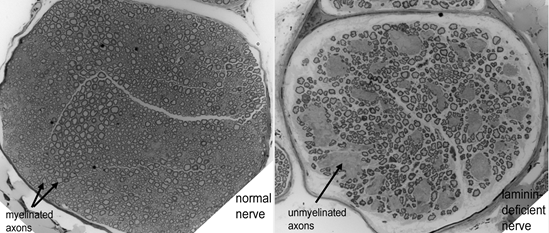
Fig. above: Cross-sections of sciatic nerve from a normal mouse (left) and a laminin-hypomorphic mouse (right). Note groups of unmyelinated axons in nerve on right.
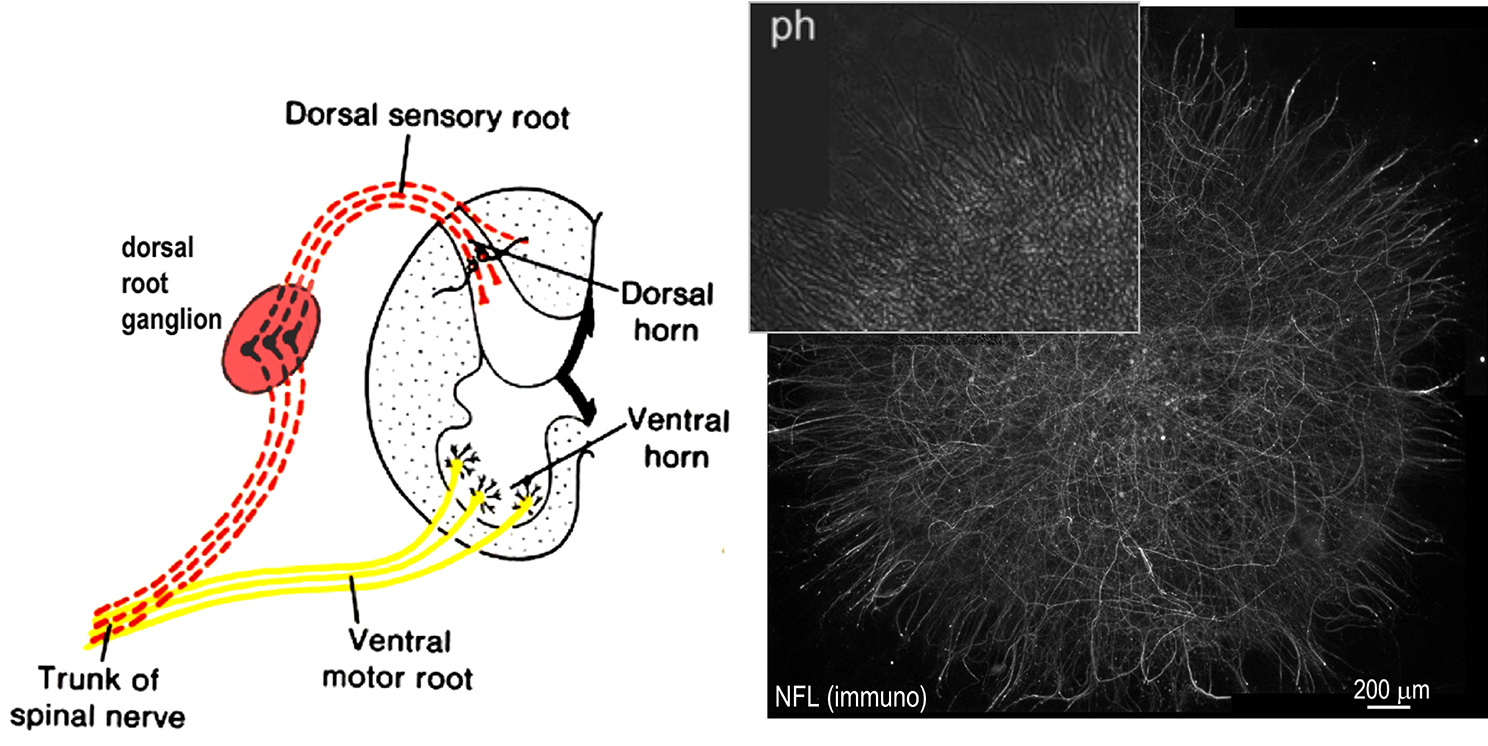
Fig above: Dorsal root ganglia (DRG) are collections of sensory nerve bodies, their axons and Schwann cells located posterolateral to the spinal cord. These can be isolated from embryonic mice and grown in culture, allowing one to follow the process of axonal myelination. A phase image (ph) and immunofluorescence image of neurofilament protein (NFL, axonal marker) of such a culture is shown to the right. Laminin expression is prevented by isolating laminin alpha2/alpha4 double null DRGs or cre-inactivated LAMC1 flox/flox DRGs. Myelination and Schwann cell proliferation is induced by adding recombinant laminins, nidogen and ascorbic acid (needed for type IV collagen).
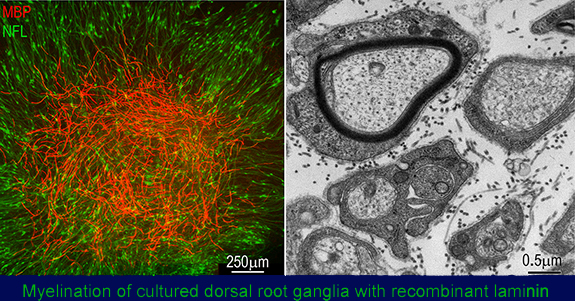
Fig. above: Left: immunofluorescence light micrograph image of a laminin-deficient DRG in which myelination (stained red, myelin basic protein, MBP) induced with recombinant laminin-111 is shown in relationship to the axons (NFL, green). Right: electron micrograph showning a myelinated axon (above) adjacent to sorted axons, with each Schwann cell completely surrounded by thin basal laminae.
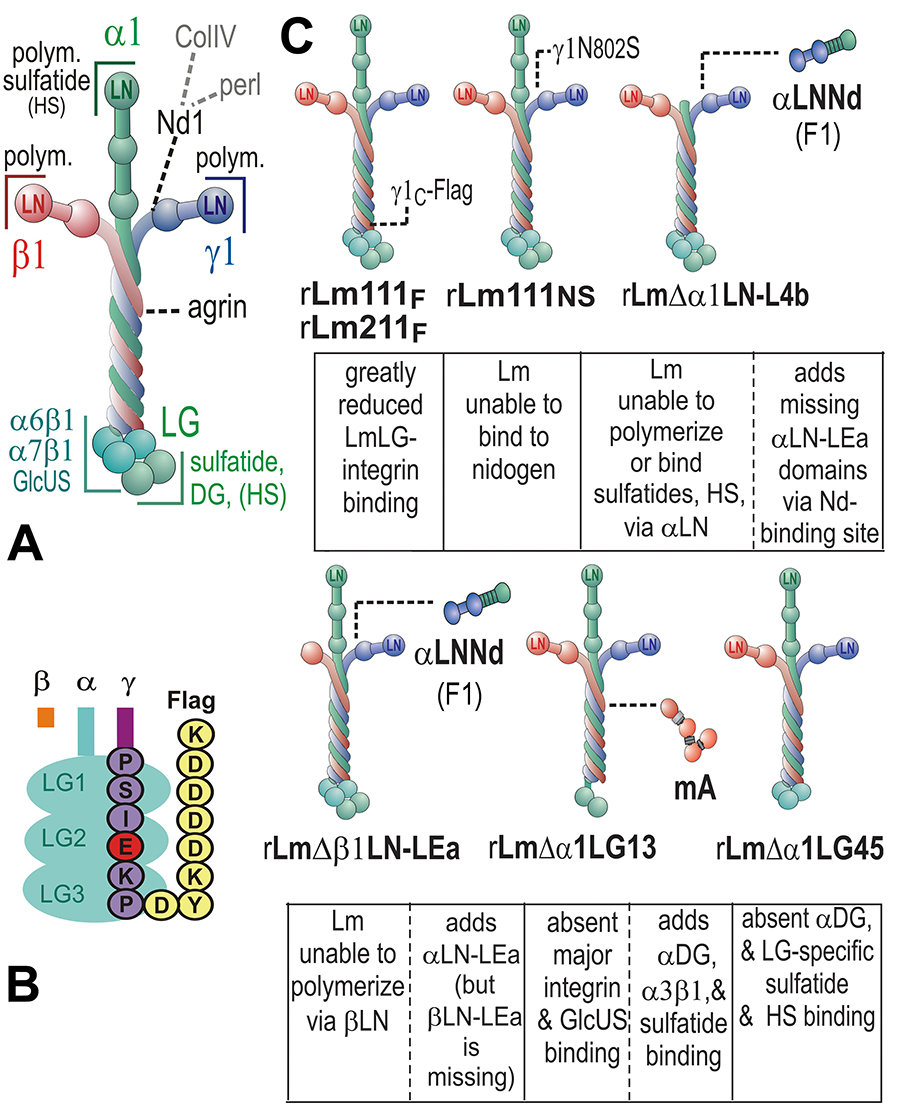
Fig. above: These are modifications of recombinant laminins used to determine the domains and their activities required for myelination and Schwann cell proliferation.
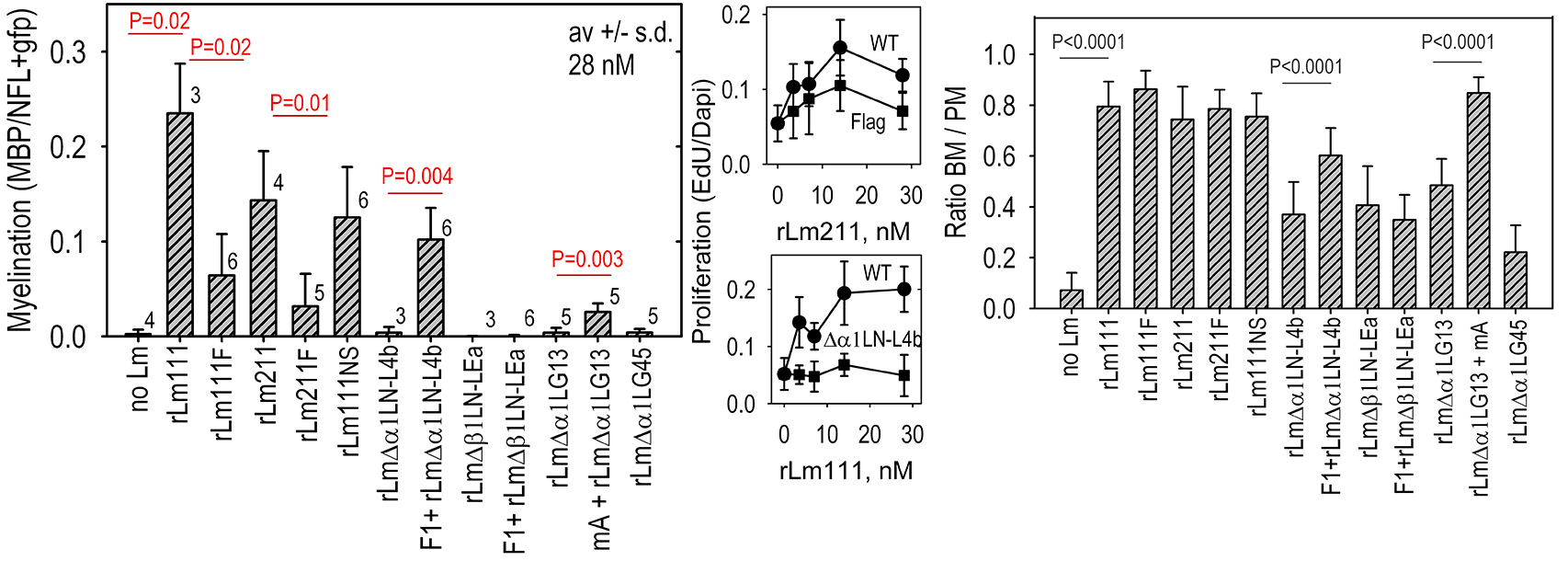
Fig. above: Applications of the different recombinant laminins revealed that (a) myelination required laminin polymerization as well as adhesion through LG domains(especially integrin-binding activity), that proliferation depended upon laminin polymerization (repairable with F1) and integrin-mediated interactions through LG domains, and that BL assembly required laminin polymerization but not LG-mediated integrin binding.
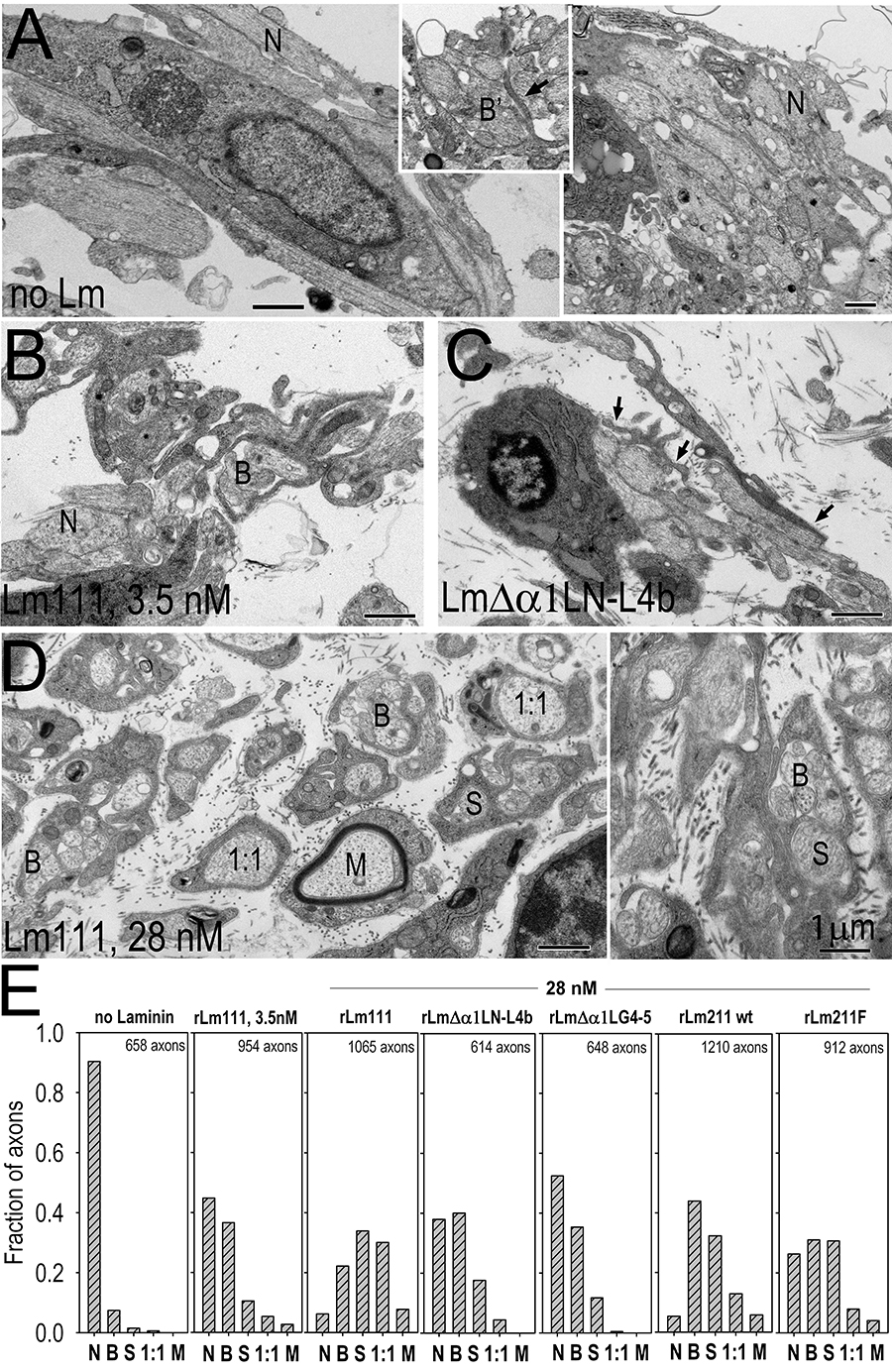
Fig. above: Radial axonal sorting in laminin-deficient dorsal root ganglia cultures following induction with different recombinant laminins. Adeno-cre virus treated Lamc1-fl/fl DRGs were denied laminin (A, no laminin), treated with 3.5 nM wild-type rLm111 (B), 28 nM non-polymerizing rLmDaLN-L4b (C), or 28 nM wild-type rLm111 (D), all in the presence of 28 nM nidogen-1 and in the presence of ascorbate for six days. In the absence of added laminin, most axons were naked (N) and often grouped in large bundles (right side). While some of the axons were in direct contact with SCs, they were rarely surrounded by cell processes (when such processes were observed (inset), the processes were usually of short length resulting in incomplete bundling (B’). At low concentrations of laminin, many of the axons were noted to be partially (one-half to two-thirds) grouped into bundles by lamellipodia (B’) or more fully grouped (B), and with a substantially increased fraction of axons sorted (S) within cells and cell processes, and located fully and individually within Schwann cells (1:1), with infrequent axonal myelination (M). At high concentration of laminin (H, two frames), naked axons were only occasionally encountered. In contrast, substantial fractions of sorted and 1:1 segregated axons were present. Panel I. Plots of the distribution of naked (N), bundled (B, includes B’), sorted (S) and myelinated (M) axons as described were determined by from multiple micrographs. The highest degree of sorting was detected following treatment with 28 nM laminin-111, in marked contrast to no treatment. Treatment with wild-type rLm211 considerable sorting; however, this was less-advanced compared to Lm111. The distribution of sorted axons was further reduced with Flag-tag modified rLm211. Laminin unable to polymerize (rLmΔα1LN-L4b) or lacking LG4-5 cell-adhesion domains (rLmΔα1LG4-5) had a considerable fraction of naked axons; however, it exhibited prominent bundling and moderate fractions of sorted axons, unlike the no-treatment condition.