In order to understand the functions of laminins in the renal collecting system, the LamC1 gene was inactivated in the developing mouse ureteric bud (UB). Embryos bearing null alleles exhibited laminin deficiency prior to mesenchymal tubular induction and either failed to develop a UB with involution of the mesenchyme, or developed small kidneys with decreased proliferation and branching, delayed renal vesicle formation, and post-natal emergence of a water transport deficit. Embryonic day 12.5 kidneys revealed nearly-absent basement membrane proteins and reduced a6-integrin and FGF2. mRNA levels for fibroblast growth factor-2 (FGF2), and mediators of the GDNF/RET and Wnt-11 signaling pathway were also decreased. Furthermore, collecting duct cells derived from laminin-deficient kidneys and grown in collagen gels were found to proliferate and branch slowly. The laminin-deficient cells exhibited decreased activation of growth factor- and integrin-dependent pathways whereas heparin lyase-treated and b1 integrin-null cells exhibited more selective decreases. Collectively the data support a requirement of g1-laminins for assembly of the collecting duct system basement membrane whose immobilized ligands act as solid-phase agonists to promote branching morphogenesis, growth and water transport functions

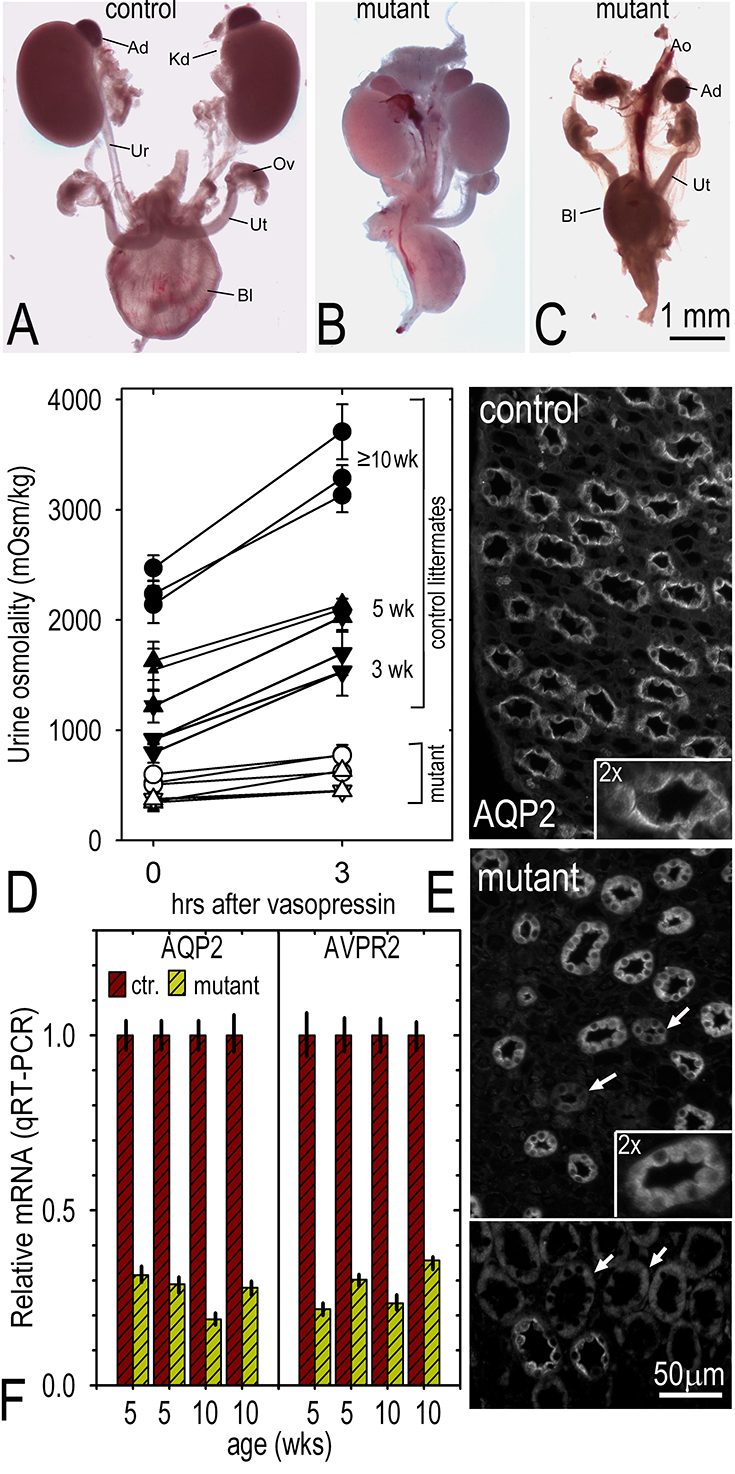
Fig. above: Postnatal phenotypes. Panels A-C. P0. Kidneys (Kd), adrenal glands (Ad), ureters (Ur), bladder (Bl), ovaries (Ov) and uterus (Ut) with portions of abdominal aorta (Ao) were dissected from newborn (P0-P1) heterozygous (A) and homozygous mutant (B, C) female pups. Mutant mice either possessed kidneys, both small with normal appearing ureters and bladder, or lacked both kidneys and ureters with empty bladders. Panels D-F. Postnatal urine concentration defect. Panel D. Plot of urine osmolality (average and S.D. of 3 consecutive measurements per mouse) before and after vasopressin treatment of laminin-deficient (solid symbols) and control (open symbols) littermates at 3 weeks (inverted triangles), 5 weeks (triangles) and ≥ 10 weeks (circles) of age. E. Aquaporin-2 immunostaining in adult control and laminin-deficient renal papillae (latter shown from two fields, one an overlapping composite ; arrows indicate weakly staining collecting ducts). Collecting ducts from control papillae had bright apical distributions of aquaporin-2. Ducts from mutant papillae had an altered pan-intracellular distribution (arrows) of aquaporin-2 that was often reduced in intensity. F. Quantitative RT-PCR determinations for aquaporin-2 (AQP2) and the vasopressin-2 receptor (V2R) mRNA extracted from four control and four laminin-deficient kidneys.