INTRODUCTION
Basal laminae (BL, also known as basement membranes) are evolutionarily ancient cell-adherent extracellular matrices (ECMs) that are required for embryonic development and for normal tissue functions. They are sheet-like scaffolds that assemble under epithelium and vascular endothelium, and around muscle, peripheral nerve Schwann cells, and adipocytes. Mutations affecting the structural proteins of these ECMs (laminins, collagen, nidogen and proteoglycans) cause diseases of muscle, kidney, nerve, brain, eye and skin. Engineered mutations of basal lamina components result in developmental and post-developmental defects with failures of gastrulation, organogenesis and/or specific functions of kidney, skin, vessels, nerve, muscle, brain, eye, placenta and other tissues. In the adult, post-developmental alterations of basal lamina architecture and function contribute the the pathogenesis of diabetes mellitus, altering vascular permeability.
The long-term goals of the laboratory are to understand how basal laminae assemble, how structure dictates function, how basal laminae influence cell behavior at the molecular level, how defects of assembly cause diseases, and how the defects can be repaired. The experimental focus is on basal laminae found in early embryos, kidney, muscle and nerve. The methods used are those of molecular biology, cell biology and mouse genetics.
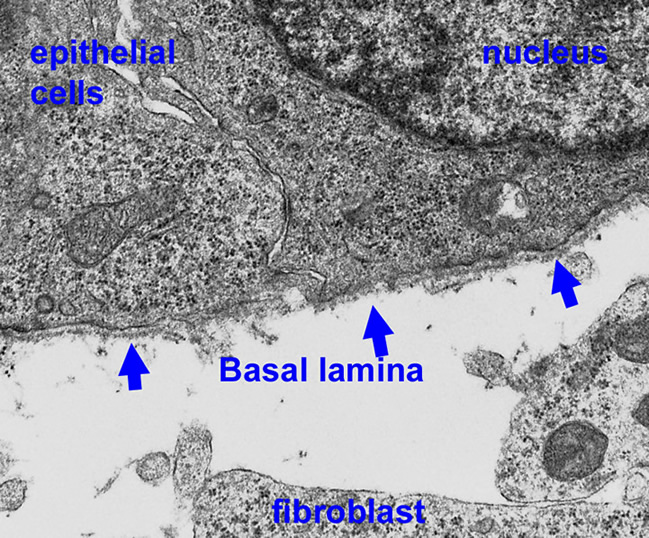
General functions: Basal lamina ECMs are solid-phase cell substrates and agonists that act as (1) inducers of differentiation and (2) critical separators of cell types within a developing tissue. In addition they (3) provide architectural support for cell layers and groups, (4) form permselective barriers between cell compartments, and (5) serve as migration surfaces for cells during development and regeneration following injury.
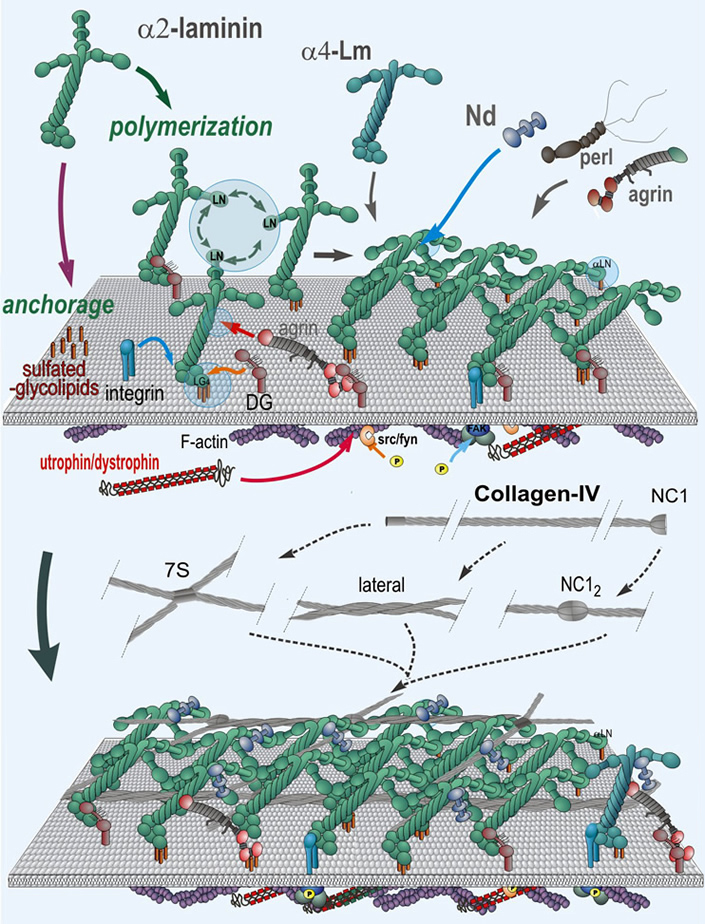
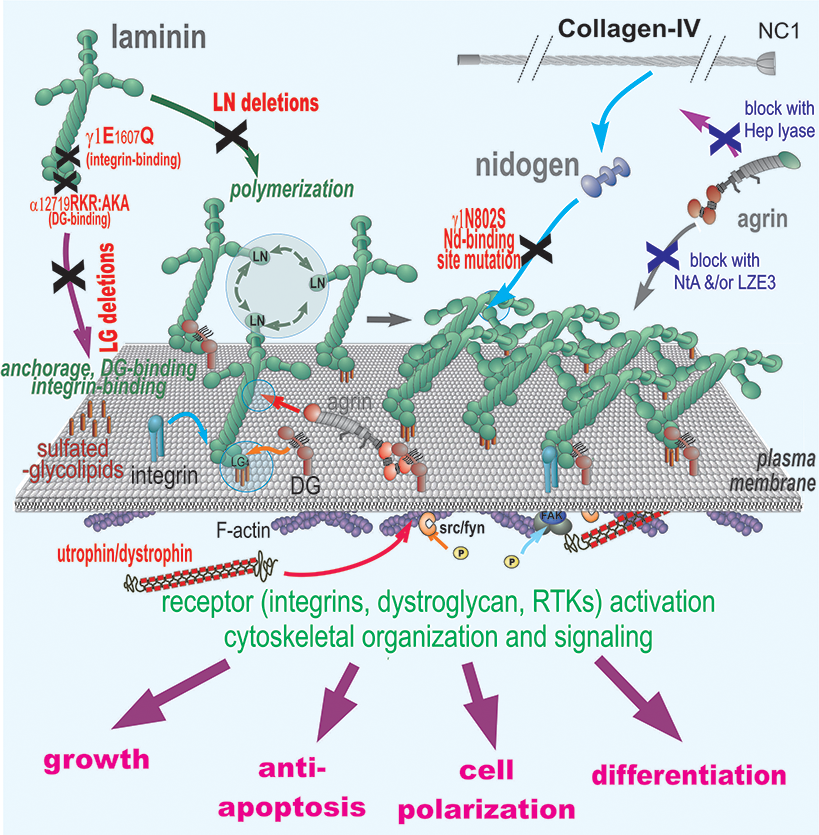
Matrix assembly and stabilization during development: The minimal requirement for assembly of a basal lamina within a tissue appears to be that of polymerization of a suitable laminin and anchorage of that laminin to an appropriate cell surface. Stability requires incorporation of type IV collagen which forms a second network, nidogen and heparan sulfate proteoglycans that bridge laminin polymer to the type IV collagen network, and sufficient anchorage through receptors and other cell surface binding interactions.
Specialization of functions: There are at least fifteen laminins (each a heterotrimer joined through a coiled-coil domain), three type IV collagens (each a heterotrimeric triple helix), and two nidogens (entactins) found in different basement membranes. As tissues differentiate, switches occur in the expression of laminin isoforms.
Receptor Interactions: Basal lamina components interact with receptors that transduce signals to the cell interior. Interacting receptors include integrins, receptor kinases and dystroglycan. Receptor engagement of polymerized ligands can affect growth, cell phenotype, and migratory behavior.
Stem cells and early embryogenesis: Basal laminae first form before implantation (mouse embryonic ~ day 4). These ECMs (that of the visceral endoderm/epiblast and parietal endoderm/trophectoderm) appear to be initiated by the secretion and polymerization of laminin-111 and -511 which form a critical first matrix scaffolding. Laminin is essential for initiation of gastrulation and plays a critical role in determining the differentiating fate of embryonic stem cells.
Neuromuscular axis: Defects in laminins bearing the alpha2 subunit cause disorders of skeletal muscle, neuromuscular junction (NMJ), peripheral nerve and brain. Indeed, more than half of cases of human congenital muscular dystrophy (CMD) are due to mutations in or loss of the laminin-alpha2 subunit. This disorder is characterized by severe and progressive early onset muscle weakness and white matter defects of the brain. The pathology of the muscle, NMJ and nerve is one of muscle necrosis and regeneration, small NMJs with reduce infoldings, and a radial sorting defect of Schwann cells in nerve in which the Schwann cells fail to divide naked axons, leaving them without myelination.
Kidney morphogenesis. Basal laminae play key roles in the morphogenesis of kidney glomeruli, tubules, and vasculature. We have found that laminins are critical for the maintenance of collecting duct cell polarity, while the heparan sulfates of the basement membrane are needed to present growth factors such as FGF to the tubular cells to promote their proliferation and branching. These growth factors act in concert with integrins.